Gd(OH)3 with multiform morphologies and MRI contrast agent properties by different solvents
Abstract
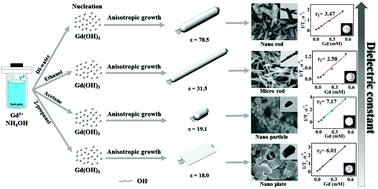
Gd(OH)3 nanocrystals with rod, particle and plate morphologies have been synthesized for drug delivery and MRI imaging. Structural analysis revealed Gd(OH)3 nanocrystals to be of the single crystal hexagonal structure. The morphological and structural properties of samples were evaluated by scanning electron microscopy, transmission electron microscopy and MR imaging. Their size and morphology were controlled by varying the surface energy, solubility, nucleation density and dielectric constant of solvents. The one-, two-, and three-dimensional structures affect materials to change physical, chemical and magnetic properties. The solvents were found to have a crucial influence on the growth mechanism, morphology and size of the Gd(OH)3 particles because of the various interactions with a host. Therefore, the MRI signal intensity of these nanocrystals was also affected. They have 3.47 mM−1 s−1 (DI-water 40 mL, nanorod), 2.58 mM−1 s−1 (ethanol 40 mL, microrod), 7.17 mM−1 s−1 (acetone 40 mL, nanoparticle), 6.01 mM−1 s−1 (2-propanol 40 mL, nanoplate). The process is very simple and scalable. In addition, it can be used for the fundamental studies of tunable sizes and morphology formation which influence the MRI signal intensity.

 Please wait while we load your content...
Please wait while we load your content...