Evolution from linear tetranuclear clusters into one-dimensional chains of Dy(iii) single-molecule magnets with an enhanced energy barrier†
Abstract
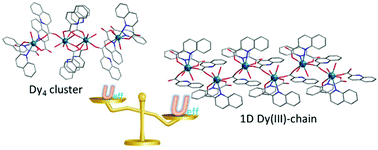
Reactions of DyCl3·6H2O or Dy2O3 with 2-quinolinecarboxylic acid (H-QLC) yielded two compounds, namely, [Dy4(QLC)12(H2O)6]·4H2O (1) and [Dy(QLC)3(H2O)2]n (2). Compound 1 is a linear tetranuclear structure with one central [Dy2(QLC)4(H2O)4] subunit and two terminal [Dy(QLC)3(H2O)] subunits linked by bridging QLC− ligands. Compound 2 has a one-dimensional (1D) chain wherein the [Dy(QLC)2] unit formed by two QLC− ligands chelating a Dy(III) ion is connected by the bridging QLC− ligands. Compounds 1 and 2 exhibit slow magnetic relaxation behaviour in the absence of a static magnetic field, which is rarely observed in lanthanide-carboxylate compounds. During the structural evolution from a linear Dy4 cluster of 1 into a 1D chain of 2, the anisotropy energy barrier (Ueff) is enhanced from 45.4(2) to 144.2(1) K. Though the Dy(III) centers in 1 and 2 all are eight-coordinated with square antiprismatic coordination environments, the higher energy barrier observed for 2 could be the result of a more favorable crystal field for the Dy(III) ions in 2.


 Please wait while we load your content...
Please wait while we load your content...