Mechanisms of neptunium redox reactions in nitric acid solutions†
Abstract
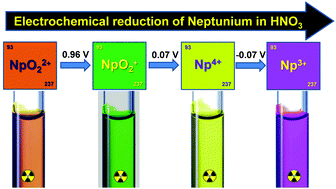
The first transuranium element neptunium (Np) exhibits complicated behavior in acidic solutions as it adopts a wide range of oxidation states typically from +3 to +6 and coordinates with a large variety of ligands. In particular, the accurate determination of Np redox potentials in nitric acid solutions is challenging due to overlapping chemical and electrochemical reactions leading to significant experimental uncertainties. Over the past several decades, spectrophotometry has been extensively applied to identify and characterize Np solution species in different oxidation states. However, relevant spectral databases of Np in nitric acid solutions that can serve for reference purposes are yet to be established due to the experimental difficulty in isolating and stabilizing Np species in pure oxidation states without compromising their solution optical properties. This work demonstrates that a combination of voltammetry and controlled-potential in situ thin-layer spectropotentiometry overcomes these challenges, and vis–NIR spectra of electrochemically generated Np species in pure +3, +4, +5, or +6 oxidation states in the systematically varied 0.5–4 M nitric acid solutions were obtained. In situ optical monitoring of the interconversion between adjacent Np oxidation states resulted in elucidation of the mechanisms of the involved redox reactions and an in-depth understanding of the relative stability of the Np oxidation states, and allowed benchmarking of the redox potentials of the NpO22+/NpO2+, NpO2+/Np4+ and Np4+/Np3+ couples. Notably, the NpO2+/Np4+ couple was distinguished from the proximal Np4+/Np3+ process overcoming previous concerns and challenges encountered in the accurate determination of the respective potentials.


 Please wait while we load your content...
Please wait while we load your content...