Isoselective mechanism of the ring-opening polymerization of rac-lactide catalyzed by chiral potassium binolates†
Abstract
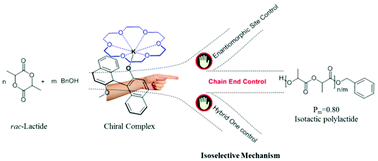
In this work, two enantiopure potassium complexes were synthesized and characterized. These two complexes show good isoselectivity for the ring-opening polymerization (ROP) of rac-lactide (Pm = 0.80 at room temperature for complex 1 and Pm = 0.80 at −60 °C for complex 2) and display very different activities for the ROP of rac-lactide due to the presence of an intramolecular hydrogen bond in complex 1. Meanwhile, a ligand assisted monomer activated mechanism for the ROP of lactide is rationalized in the presence of alcohol for this system. The stereoselective mechanism is ascribed to a chain end control mechanism via three experimental methods (the analysis of the stereo microstructure of the polymer, the activity of one enantiopure catalyst towards the ROP of L-lactide, D-lactide, and rac-lactide, and the enantiomeric excess values of the residual monomers and polymers via optical rotation measurements at different ROP conversions).


 Please wait while we load your content...
Please wait while we load your content...