The effect of magnetic coupling on magneto-caloric behaviour in two 3D Gd(iii)–glycolate coordination polymers†
Abstract
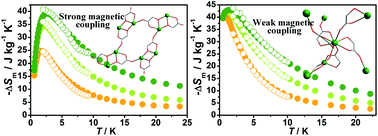
Two different 3D coordination polymers, [Gd(glc)(Hglc)(H2O)]n·nH2O (1) and [Gd(Hglc)3]n (2) (H2glc = glycolic acid), have been prepared based on the reaction of Gd(III) ions with H2glc in different pH environments. Due to the deprotonation of the α-OH groups from half of the H2glc ligands in 1, [Gd2] units with a Gd–O–Gd bridging model are observed and suggest a stronger magnetic coupling than that in 2. Magnetic and heat capacity studies reveal the significant impact of the strength of magnetic coupling on the magneto-caloric effect (MCE) in systems. Although a theoretical calculation suggests the −ΔSm (50.5 J kg−1 K−1) of 1 is larger than 2 (45.2 J kg−1 K−1), the stronger antiferromagnetic coupling in 1 decreases the number of low-lying excited spin states upon the lowering of temperature, thus giving a smaller value of −ΔSm than 2 at various fields (e.g. −ΔSm,max = 24.8 J kg−1 K−1 and 41.1 J kg−1 K−1 for 1 and 2 respectively at ΔH = 3 T). This case reveals that the effect of magnetic coupling on MCE plays a dominant role for designing low-temperature 3D Gd(III)-based magnetic coolants.

 Please wait while we load your content...
Please wait while we load your content...