Theoretical studies on tricarbonyl metal derivatives of Lindqvist-type polyoxometalate complexes: electronic structures and nonlinear optical properties
Abstract
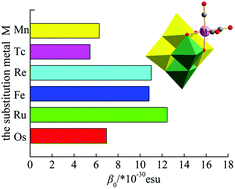
The density functional theory (DFT) method and natural bond orbital (NBO) analysis have been employed to investigate the electronic structures and second-order nonlinear optical (NLO) properties for tricarbonyl metal derivatives of a Lindqvist-type polyoxometalate, [Nb2W4O19M(CO)3]3−/2− (M = MnI, TcI, ReI, FeII, RuII and OsII). The position of the tricarbonyl metal ligand and the substitution of different metal atoms M have an influence on the electronic absorption spectra and NLO responses of all complexes. Among the three isomers of [Nb2W4O19Mn(CO)3]3−, the β0 value of isomer 1a is the largest, which is 1.4 times and 2.3 times larger than those of isomers 1b and 1c, respectively. The β0 value also depends on the tricarbonyl metal M, the β0 value decreases in the order M = Ru > Re > Fe > Os > Mn > Tc. In addition, the analysis of the main transition orbitals shows that the tricarbonyl metal ligand acts as an electron donor in [Nb2W4O19M(CO)3]3−/2− (M = MnI, TcI, ReI, RuII and OsII), while the [Fe(CO)3]2+ ligand acts as an electron acceptor in [Nb2W4O19Fe(CO)3]2−.

 Please wait while we load your content...
Please wait while we load your content...