Syntheses, structures and gas sorption properties of two coordination polymers with a unique type of supramolecular isomerism†
Abstract
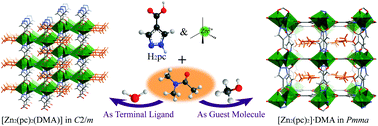
The solvothermal reaction of a short bridging ligand 1H-pyrazole-4-carboxylic acid (H2pc) and Zn(NO3)2 in a mixed solvent containing N,N-dimethylacetamide (DMA) at 110 °C produced two genuine supramolecular isomers [Zn2(pc)2(DMA)] (1) and [Zn2(pc)2]·DMA (2). Single-crystal X-ray diffraction studies showed that 1 is a densely packed layered structure with DMA molecules coordinated on the layered surface, and 2 is a porous three-dimensional framework structure with DMA molecules filled inside the pore without coordination to any metal ion, which represents a rare case of coordination-sphere isomerism in coordination polymers. Removal of the coordinated DMA molecules in 1 occurs at high temperatures, accompanying the irreversible formation of an unidentified nonporous phase. On the other hand, the guest DMA molecules in 2 can be readily removed under mild conditions to give a new porous phase [Zn2(pc)2] (2′). Gas-sorption measurements of 2′ revealed that the framework has significant flexibility and selective CO2 adsorption at room temperature.
- This article is part of the themed collection: Crystal engineering for molecular materials

 Please wait while we load your content...
Please wait while we load your content...