On the flexibility of carboranylalkylthio substituents in porphyrazines and its relevance to the photophysical properties†
Abstract
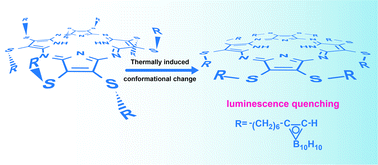
Ultrafast transient absorption spectrometry and DFT/TDDFT calculations reveal that following photoexcitation, carboranyl- and carboranyl-free alkylthioporphyrazines deactivate by the pathway S1(π,π*)→Sn(Cβ-2pz/Sl.p.,π*)→ground state. The presence of quenching singlet excited states with predominantly Cβ-2pz/Sl.p.,π* character immediately below the primarily photogenerated S1(π,π*) state is a consequence of the electronic structure changes induced by the inherent flexibility of the alkylthio chains.

 Please wait while we load your content...
Please wait while we load your content...