Oxidovanadium(iv), oxidomolybdenum(vi) and cobalt(iii) complexes of o-phenylenediamine derivatives: oxidative dehydrogenation and photoluminescence†
Abstract
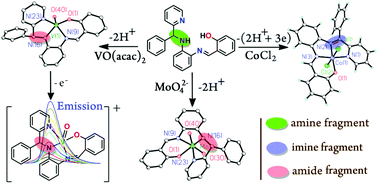
Reactions of o-phenylenediamine derivatives (L3H2) incorporating a (Ph)(Py)(H)C–N(H)– function with the oxidovanadium(IV) and oxidomolybdenum(VI) ions afford amide complexes of types [VIVO(L32−)] (3), [VIVO(L3t-Bu 2−)] (4) and cis-[MoVIO2(L32−)] (5) (L3H2 = ((E)-2-(((2-((phenyl(pyridin-2-yl)methyl)amino)phenyl)imino)methyl)phenol); L3t-BuH = ((E)-2,4-di-tert-butyl-6-(((2-((phenyl(pyridin-2-yl)methyl)amino)phenyl)imino)methyl)phenol)), while the similar reaction of L3H2 with the anhydrous CoCl2 in air results in oxidative dehydrogenation (OD) of the (Ph)(Py)(H)C–N(H)– function, affording a cobalt(III) diimine complex, trans-[CoIII(L4−)Cl2] (6) (L4H = 2-((E)-(2-((E)-phenyl(pyridin-2-yl)methyleneamino)phenylimino) methyl)phenol), contradicting the participation of the higher oxidation states of the metal ions in OD reaction of amines. 3–6 are characterized by elemental analyses and mass, IR, 1H NMR and EPR spectra. The molecular geometries of 4·CH3OH, 5 and 6 were confirmed by single crystal X-ray structure determinations. The VIV–Ophenolatocis to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the VIV
O bond and the VIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O lengths in 4·CH3OH are 1.925(2) and 1.612(2) Å. Two cis Mo
O lengths in 4·CH3OH are 1.925(2) and 1.612(2) Å. Two cis Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O lengths are 1.710(2) Å and 1.720(2) Å in 5. The aliphatic –C–N– lengths in 4·CH3OH and 5 are 1.448(3) and 1.479(2) Å, while the same is 1.285(4) Å in 6. DFT calculations on 3 and 6 inferred a significant mixing among dM and NN-ligand backbone favoring a t26 state of the metal ion for the OD of the amine fragment to have stronger dM → πketimine* back-bonding. The πNHPh → πaldimine* transition of L3H2 is red shifted in 3 and 4 quenching the emissive πPhenolato → πaldimine* transitions, elucidated by the TD DFT calculations on 3 (and 3+). The πNPh → πaldimine* transitions are blue shifted in the oxidovanadium(V) analogues, [VVO(L32−)]+ (3+) and [VVO(L3t-Bu 2−)]+ (4+), which are fluorescent (3+, λex = 331, λem = 444 nm; 4+, λex = 339, λem = 490 nm) recorded by the fluorescence-spectroelectrochemical measurements in CH2Cl2. 5 and 6 emit weakly at 466 and 473 nm (5, λex = 336 nm, ϕ = 0.003; 6, λex = 324 nm, ϕ = 0.027).
O lengths are 1.710(2) Å and 1.720(2) Å in 5. The aliphatic –C–N– lengths in 4·CH3OH and 5 are 1.448(3) and 1.479(2) Å, while the same is 1.285(4) Å in 6. DFT calculations on 3 and 6 inferred a significant mixing among dM and NN-ligand backbone favoring a t26 state of the metal ion for the OD of the amine fragment to have stronger dM → πketimine* back-bonding. The πNHPh → πaldimine* transition of L3H2 is red shifted in 3 and 4 quenching the emissive πPhenolato → πaldimine* transitions, elucidated by the TD DFT calculations on 3 (and 3+). The πNPh → πaldimine* transitions are blue shifted in the oxidovanadium(V) analogues, [VVO(L32−)]+ (3+) and [VVO(L3t-Bu 2−)]+ (4+), which are fluorescent (3+, λex = 331, λem = 444 nm; 4+, λex = 339, λem = 490 nm) recorded by the fluorescence-spectroelectrochemical measurements in CH2Cl2. 5 and 6 emit weakly at 466 and 473 nm (5, λex = 336 nm, ϕ = 0.003; 6, λex = 324 nm, ϕ = 0.027).

 Please wait while we load your content...
Please wait while we load your content...