Nanosized zinc peroxide (ZnO2): a novel inorganic oxidant for the oxidation of aromatic alcohols to carbonyl compounds
Abstract
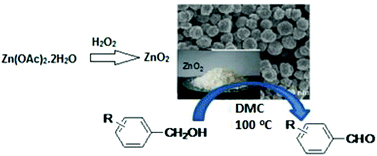
Zinc peroxide (ZnO2) nanoparticles were readily synthesized from the oxidation of zinc acetate with hydrogen peroxide at room temperature. The synthesized material was fully characterized by FTIR, XRD, TG-DSC, FE-SEM, TEM and XPS analysis. As obtained nanosized zinc peroxide was found to be an efficient oxidant for the oxidation of aromatic alcohols to the corresponding carbonyl compounds selectively in excellent yields using dimethyl carbonate as an environmentally benign solvent.

 Please wait while we load your content...
Please wait while we load your content...