Highly 2,3-selective and fast living polymerization of alkyl-, alkoxy- and phenylallenes using nickel(ii) catalysts†
Abstract
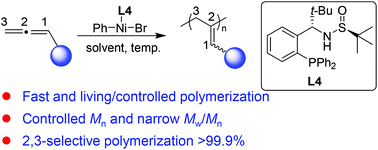
Polyallene is one of the most attractive synthetic precursors for constructing functional materials due to its unsaturated bonds on the main chain or pendants. The facile synthesis of high regioselectivity polyallenes still remains a challenge. In this work, a novel Ni(II) catalyst with a phosphine bearing sulfoxide moiety named Ming-Phos (L4) as a ligand was developed to initiate the polymerization of various allene monomers efficiently in a fast and living/controlled manner. The well-defined polyallenes were afforded in high yields with a controlled molar mass (Mn) and low molar mass distribution (Mw/Mn). Moreover, the Ni(II)/L4 catalyst exhibited fascinating regioselectivity on the accumulated double bonds of allene monomers, and the 2,3-regioselectivity of the polymerization of allene monomers was up to >99.9%. Remarkably, various monomers with different functional groups inculding alkyl, alkoxy and substituted phenyl groups could be polymerized effectively using Ni(II)/L4 as the initiator, affording polyallenes with higher 2,3-regioselectivity. The regioselectivity, and thermodynamic and crystallization properties of the polyallenes were confirmed by 1H NMR, DSC, TGA and WAXD, respectively.


 Please wait while we load your content...
Please wait while we load your content...