Temperature-mediated molecular ladder self-assembly employing Diels–Alder cycloaddition†
Abstract
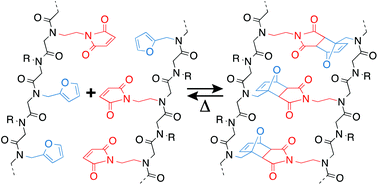
Dynamic covalent self-assembly processes often exhibit poor capacities for error-correction owing to the relatively low connectivity rearrangement rates of dynamic covalent interactions and the common use of reaction conditions where the equilibrium state remains fixed. Here, we report a dynamic covalent self-assembly technique employing temperature, a conventional, externally-applied stimulus, to mediate the hybridization of peptoid oligomers bearing maleimide- and furan-based pendant groups to afford molecular ladders incorporating Diels–Alder adduct-based rungs. By raising or lowing the reaction temperature, this system enables the equilibrium state to be readily varied without altering reagent concentrations. Both triethylamine and the Lewis acidic scandium triflate were examined as candidate reaction catalysts; however, only scandium triflate increased the rate of single strand conversion. As the Diels–Alder cycloaddition reaction does not liberate a small molecule, a registry-dependent mass change was effected by employing a base-catalyzed thiol-Michael addition reaction between any un-reacted maleimide pendant groups and a low molecular weight thiol to enable the number of Diels–Alder adduct rungs to be readily determined by mass spectrometry. Finally, by employing a slow temperature ramp from high to low temperature, approximating the thermal cycle employed for nucleic acid hybridization, sequence-selective hybridization between model, tetra-functional precursor strands was demonstrated.
- This article is part of the themed collection: Polymer Chemistry Pioneering Investigators 2021


 Please wait while we load your content...
Please wait while we load your content...
