Rational molecular design of anion exchange membranes functionalized with alicyclic quaternary ammonium cations
Abstract
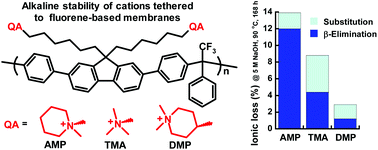
High alkaline stability is critical for polymeric anion exchange membranes (AEMs) and ionomers for use in alkaline electrochemical energy conversion and storage devices such as fuel cells, electrolyzer cells and advanced batteries. Here, we have prepared and studied ether-free polyfluorenes tethered with N,N-dimethylpiperidinium (DMP) and 6-azonia-spiro[5.5]undecane (ASU) cations, respectively, attached through heteroatom-free alkyl spacers. By employing alkyl–alkyl Suzuki cross-coupling, these alicyclic quaternary ammonium cations are attached at the 4-position to impede ionic loss. Thus, all the β-hydrogens sensitive to elimination reactions are placed in strain-free rings able to fully relax by the spacer flexibility. Consequently, the AEM carrying DMP cations shows a very high alkaline and thermal stability, retaining more than 91% of the cations after 2400 h immersion in 2 M NaOH at 90 °C. Compared with corresponding AEM functionalized with N-alkyl-N-methylpiperidinium (AMP) cations [conventionally tethered via the 1(N)-position], the ionic loss by β-elimination is successfully reduced by up to 92%. The AEM functionalized with DMP also reaches a high hydroxide conductivity of 124 mS cm−1 at 80 °C. Consequently, tethering piperidine-based cations via the 4-position instead of the 1(N)-position results in AEMs with substantially improved thermal and alkaline stability, combined with high hydroxide conductivity.


 Please wait while we load your content...
Please wait while we load your content...