Saccharin-pendant methacrylamide as a unique monomer in radical copolymerization: peculiar alternating copolymerization with styrene†
Abstract
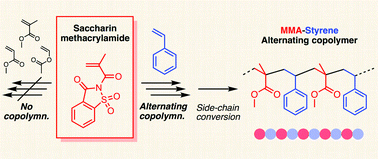
In this article, we report an unusual reactivity of saccharin-pendant methacrylamide (1) in radical copolymerization. The bulky vinylidene molecule showed no homopolymerization ability under general radical polymerization conditions likely due to the bulky pendant for the inherently low reactivity monomer of N,N-disubstituted methacrylamide. However, in the presence of styrene, it was smoothly consumed at the same rate as styrene. Given the near-zero monomer reactivity ratios of the pair [r1 = 0.040 and r2 = 0.045 for 1 (M1) and styrene (M2)], it was assumed that the comonomer pair afforded alternating copolymerization. The copolymerization was very specific to the combination since copolymerizations of 1 with other conjugated monomers such as methacrylate and acrylate did not progress. The peculiar copolymerization reactivity of 1 was supported by DFT calculations. The side chains of 1 in the alternating copolymer were converted into methyl ester via hydrolysis and methylation, and thus an alternating copolymer of methyl methacrylate and styrene was obtained, and the alternating sequence was characterized by 1H and 13C NMR.


 Please wait while we load your content...
Please wait while we load your content...