Stimuli-responsive non-ionic Gemini amphiphiles for drug delivery applications†
Abstract
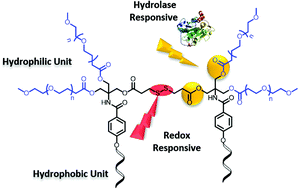
A series of six novel disulfide-bond-linked Gemini amphiphiles have been synthesized using polyethylene glycol (PEG) as hydrophilic units and alkoxy aryl moieties as hydrophobic units. The self-assembling behaviour of the resulting amphiphiles was studied using fluorescence spectroscopy and dynamic light scattering (DLS). The inner hydrophobic core with an alkyl and fluoroalkyl chain aggregates differently in aqueous medium as evidenced by a cryo-TEM study. Also they are found to be capable of encapsulating a lipophilic guest. The encapsulation behaviour was studied using Nile red as a model hydrophobic dye and extended further by including curcumin and an anti-cancerous drug doxorubicin. Furthermore, for biological evaluation, their cytotoxicity and cellular uptake were studied. The presence of stimuli responsive disulfide linkages and the ester functionality provides the possibility of redox responsive and enzymatic release of the encapsulated cargo, which was studied by using various concentrations of glutathione (GSH) and an immobilized lipase Novozym 435.


 Please wait while we load your content...
Please wait while we load your content...