Double neighbouring group participation for ultrafast exchange in phthalate monoester networks†
Abstract
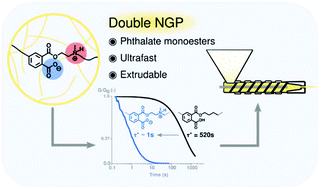
Phthalate monoesters (PMEs) were recently introduced as a simple dynamic covalent bond for implementation in covalent adaptable networks (CANs), which undergo rapid transesterifications in the absence of catalysts, due to the neighbouring group participation (NGP) of a carboxylic acid moiety. In this work, it is shown that the PME transesterification can be very significantly accelerated by the presence of another neighbouring group on the reactive alcohol moieties. The kinetic effects are demonstrated using a short model study of PMEs with different substituents at the β-carbon position, showing a remarkable acceleration for alcohols containing tertiary amines on the β-carbon. Following the model study, materials were synthesised by a (partial) replacement of the conventionally used diol with a β-amino-diol, leading to the formation of networks with an increased Tg and Young's-modulus, which is rationalised as a result of the formation of an ionic network (COO− and NHR3+). Stress relaxation experiments show a decrease in relaxation times by a factor of 500, compared to similar networks derived from non-amine-substituted hydroxyl monomers. This ultrafast relaxation, enabled by a double NGP, resulted in CANs that show potential to be processed through extrusion while maintaining their overall network connectivity.


 Please wait while we load your content...
Please wait while we load your content...