Protein aggregation nucleated by functionalized dendritic polyglycerols†
Abstract
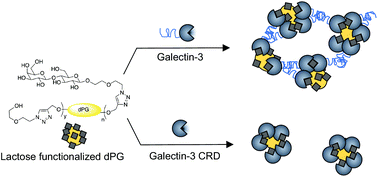
Dendritic polyglycerols (dPGs) are emerging as important polymers for the study of biological processes due to their relatively low toxicity and excellent biocompatibility. The highly branched nature and high density of endgroups make the dPGs particularly attractive frameworks for the study of multivalent interactions such as multivalent protein–carbohydrate interactions. Here, we report the synthesis of a series of lactose functionalized dPGs with different hydrodynamic radii. A series of lactose functionalized dPGs bearing different densities of lactose functional groups was also synthesized. These lactose functionalized dPGs were used to study the templated aggregation of galectin-3, a galactoside binding protein that is overexpressed during many processes involved in cancer progression. Dynamic light scattering measurements revealed a direct correlation between the hydrodynamic radii of the lactose functionalized dPGs and the size of the galectin-3/lactose functionalized dPG aggregates formed upon mixing the lactose functionalized dPGs with galectin-3 in solution. These studies exposed the critical role of galectin-3's N-terminal domain in formation of galectin-3 multimers and also enabled comparisons of polymer templated aggregation using nonspecific interactions versus specific protein–carbohydrate binding interactions.


 Please wait while we load your content...
Please wait while we load your content...
