Stereocontrolled, multi-functional sequence-defined oligomers through automated synthesis†
Abstract
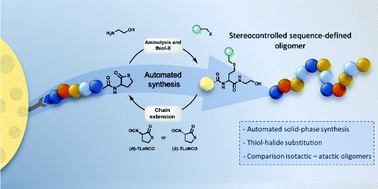
In contrast to biomacromolecules, synthetic polymers generally lack a defined monomer sequence, therefore one of the challenges of polymer chemists these days is gaining more control over the primary structure of synthetic polymers and oligomers. In this work, stereocontrolled sequence-defined oligomers were synthesised using a thiolactone-based platform. Step-wise elongation of the oligomer occurs via ring-opening of the thiolactone, resulting in the formation of stereocenters along the backbone. These initial studies indicate remarkable differences in the strength of non-covalent interactions in isotactic and atactic oligomers. Different side-chain moieties were introduced using alkyl halide building blocks and the synthetic protocol was succesfully optimised and automated. Furthermore, the possible post-synthesis modification of the oligomers was demonstrated using ‘click’ chemistry.
- This article is part of the themed collection: Molecularly Defined Polymers: Synthesis and Function


 Please wait while we load your content...
Please wait while we load your content...