Understanding the emulsion copolymerization kinetics of vinyl acetate and vinyl silanes†
Abstract
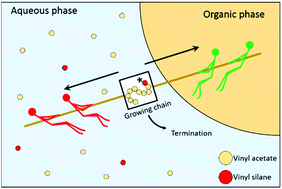
The emulsion copolymerization between vinyl acetate (VAc) and vinyl alkoxysilanes is an interesting yet not well-understood process. In the present work, the copolymerization between VAc and vinyl trimethoxysilane (VTMS) is taken as a model system and studied using different polymerization techniques in order to understand the underlying mechanism that governs the emulsion polymerization kinetics. The solution and bulk copolymerization kinetics can be explained by the reactivity ratios of the monomers determined in this work (rVTMS = 0, rVAc = 0.211). However, the emulsion copolymerization kinetics presents features that cannot be explained by the reactivity ratios, as VTMS concentrations above 5 wt% stopped the emulsion polymerization when initiated with hydrophilic initiators (such as persulfates). This was a consequence of the slow propagating radicals terminating in the aqueous phase, decreasing the concentration of surface-active radicals and thus radical entry. When more hydrophobic vinyl alkoxysilanes such as vinyl triethoxysilane are used, the inhibiting effect was less prominent, because the silane concentration in the aqueous phase was smaller. Using Density Functional Theory calculations it was found that the hydrolyzed version of the alkoxysilane monomers had a different reactivity ratio.


 Please wait while we load your content...
Please wait while we load your content...