A facile PEG/thiol-functionalized nanographene oxide carrier with an appropriate glutathione-responsive switch†
Abstract
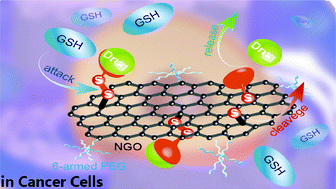
A bioreduction-responsive nanographene oxide (NGO) based drug delivery system (DDS) for chemotherapy with a disulfide linker as a glutathione-responsive switch is designed and prepared in a facile way, which can only be activated to release original water-insoluble anticancer drugs in cancer cells. For better biocompatibility and more functionalization, this DDS was constructed through a promising platform (PEG-NGO-SH), which is based on dual-functionalized NGO with six-armed poly(ethylene glycol) on the edge and thiol groups on the basal plane. After systematic experiments, this DDS was found to exhibit a suitable lateral size (180 nm), high drug loading ratio (∼20 wt%), selective release (∼90% in 10 mM GSH), bioavailability and significant difference in cytotoxicity to normal (293T) and cancer (A549) cells. The efficacy of this DDS was proved by the effective in vitro release behavior in high GSH level medium and the significant difference in cytotoxicity against A549 and 293T cells. Thus, it was obviously verified that this bioreduction-responsive DDS triggered by GSH and its construction method using a disulfide linker as a switch on dual-functionalized NGO show potency toward tumor-targeted therapy.


 Please wait while we load your content...
Please wait while we load your content...