Cyclosiloxane polymer bearing dynamic boronic acid: synthesis and bottom-up nanocoating†
Abstract
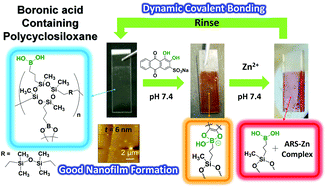
The paper describes facile synthesis of boronic ester-containing polycyclosiloxane (pCS-ABPE) through a one-pot-two-step hydrosilylation reaction that allows control of the functionalization efficiency stoichiometrically from zero to 100%. The combination of cyclosiloxane and boronic acid groups is expected to benefit from excellent thermal properties, flexible polymer backbone, and chemical resistance of the polycyclosiloxane part and the dynamic chemical properties of the boronic acid part to generate novel and functional hybrid polymers. Results showed that the hydrolysis of pCS-ABPE gave rise to a boronic-acid-containing polycylcosiloxane (pCS-AB) with good self-assembly nanofilm formation (6 nm film thickness) on plastic substrates through dip-coating method, mainly because of its low surface free energy (∼20 mN m−1). In addition, the dynaminc characteristics of boronic acid groups in the pCS-AB film were demonstrated through water soluble dye Alizarin Red S (ARS) coating. The film surface underwent reversible coating through the boronic acid equilibrium: formation of the dynamic covalent bonding (pCS-AB-ARS) and bond deformation with Zn ions for ARS-Zn complex formation. The performance makes the self-assembly nanofilm promising for chemical sensor applications.


 Please wait while we load your content...
Please wait while we load your content...