Nitroxide radical-containing polynorbornenes by ring-opening metathesis polymerization as stabilizing agents for polyolefins†
Abstract
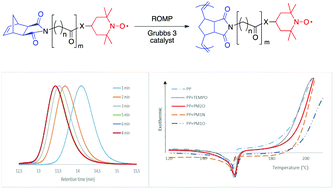
A series of original 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-containing dicarboximide norbornene monomers have been synthesized and polymerized via ring-opening metathesis polymerization (ROMP) using the Grubbs 3rd generation catalyst. First-order kinetics and linear evolutions of the molar mass with monomer conversion were obtained together with decreasing dispersity (Đ) down to 1.05–1.15. The rate of polymerization decreased with increasing alkyl chain length and an amide anchor group bound to the TEMPO functionality. Well-controlled TEMPO-containing polynorbornenes were prepared with a monomer to initiator ratio up to 1000, resulting in polymers with a high density in radical sites uniformly distributed along the backbone. These TEMPO-containing polynorbornenes have shown to be efficient as stabilizing agents in polypropylene, through onset oxidation temperature (OOT) measurements.


 Please wait while we load your content...
Please wait while we load your content...