Synthesis and characterization of a nematic fully aromatic polyester based on biphenyl 3,4′-dicarboxylic acid†
Abstract
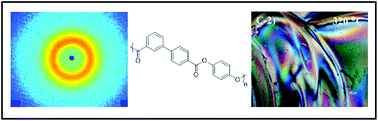
Melt acidolysis polymerization of hydroquinone with a kinked monomer, biphenyl 3,4′-bibenzoate, afforded a novel liquid crystalline polymer (LCP), poly(p-phenylene 3,4′-bibenzoate) (poly(HQ-3,4′BB)). Selection of hydroquinone diacetate (HQa) or hydroquinone dipivilate (HQp) facilitated either a tan or white final polymer, respectively. 1H NMR spectroscopy confirmed consistent polymer backbone structure for polymers synthesized with either derivative of hydroquinone. Poly(HQ-3,4′BB) exhibited the onset of weight loss at about 480 °C, similar to commercially available Vectra® LCP. Differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA) revealed a glass transition temperature (Tg) of 190 °C and an isotropic temperature (Ti) near 330 °C. The observation of a melting temperature (Tm) depended upon the thermal history of the polymer. Wide-angle X-ray scattering (WAXS) and polarized optical microscopy (POM) confirmed the formation of a nematic glass morphology after quench-cooling from the isotropic state. Subsequent annealing at 280 °C or mechanical deformation induced crystallization of the polymer. Rheological studies demonstrated similar shear thinning behavior for poly(HQ-3,4′BB) and Vectra® RD501 in the power law region at 340 °C. Zero-shear viscosity measurements indicated that HQa afforded higher melt viscosities after identical polymerization conditions relative to HQp, suggesting higher molecular weights.


 Please wait while we load your content...
Please wait while we load your content...
