Exceptional copolymerizability of o-phthalaldehyde in cationic copolymerization with vinyl monomers†
Abstract
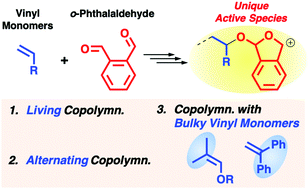
The characteristic cationic copolymerization behavior of o-phthalaldehyde (OPA) was demonstrated to allow the controlled copolymerization with an alkyl vinyl ether (VE), the alternating copolymerization, and the copolymerization with sterically hindered nonhomopolymerizable monomers such as β,β-dimethyl VE and 1,1-diphenylethylene. We first investigated the copolymerization of OPA with isobutyl VE using various initiating systems. Under the optimized conditions, the copolymerization proceeded in a living manner via the selective cyclization of two aldehydes of OPA and frequent crossover propagation reactions, and the polymerization yielded copolymers with controlled molecular weights and narrow molecular weight distributions. Moreover, β,β-dimethyl VE and 1,1-diphenylethylene, which are vinyl monomers that neither homopolymerize nor copolymerize with benzaldehyde derivatives, were successfully copolymerized with OPA to yield linear copolymers. The unique reactivity of the cyclic propagating species derived from OPA is most likely responsible for its exceptional copolymerizability.


 Please wait while we load your content...
Please wait while we load your content...