Molecularly imprinted polymers by thiol–yne chemistry: making imprinting even easier†
Abstract
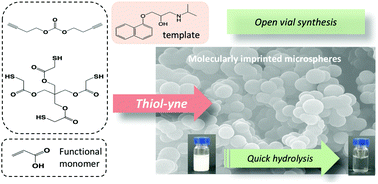
Molecularly imprinted polymers (MIPs) are synthetic materials with recognition properties comparable to those of antibodies, but with superior physico-chemical stability. Synthesized in the presence of a template, MIPs contain binding sites with high specificity and selectivity for their target. In this work, we explored the radical-mediated thiol–yne chemistry as a new approach to MIPs, using propranolol as a model template and acrylic acid as a functional monomer. Thiol–yne chemistry proved to be advantageous due to its oxygen tolerance and reactivity with (meth)acrylates. Thus, polymerization was possible in air with no need for previous deoxygenation, which is important for MIPs shaped by micro or nanofabrication. Moreover, the incorporation of ester-based polythiols made the MIPs hydrolyzable in basic and acidic conditions, thus potentially reducing their persistence in the environment. This, together with the possibility of using the well-established library of template—functional monomer(s) pairs based on (meth)acrylates, is expected to rapidly spread the use of the thiol–yne chemistry within the imprinting community.


 Please wait while we load your content...
Please wait while we load your content...