Anisotropic polymer nanoparticles with solvent and temperature dependent shape and size from triblock copolymers†
Abstract
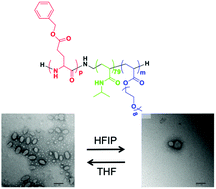
A nanoparticle system was designed able to modify its shape and size independently by the insertion of differently responsive units in a single synthetic block copolymer. The system is obtained from the self-assembly of triblock copolymers synthesized by combining reversible addition–fragmentation chain transfer (RAFT) polymerization of N-isopropylacrylamide (NIPAM) and ethylene glycol acrylate with ring-opening polymerization of γ-benzyl-L-glutamate (BLG) N-carboxyanhydride (NCA). The synthesis of triblock copolymers with different block length ratios is achieved from the poly(NIPAM) middle block followed by double chain extension including intermediate deprotection steps. All reaction products and intermediates are characterized by size exclusion chromatography (SEC) and 1H-NMR spectroscopy. Particles are obtained from triblock copolymers by solvent exchange procedures yielding either spherical or anisotropic elongated particles. The anisotropic particle shapes are confirmed by transmission electron microscopy (TEM) imaging for dry samples and multi-detector asymmetric flow field flow fractionation (AF4) of selected samples which reveals the coexistence of spherical and elongated particles in solution. Moreover, the presence of a middle poly(NIPAM) block allows to modulate the size depending on the solution temperature.


 Please wait while we load your content...
Please wait while we load your content...