Photo-responsive gels based on cyclic/linear polymers: efficient synthesis and properties†
Abstract
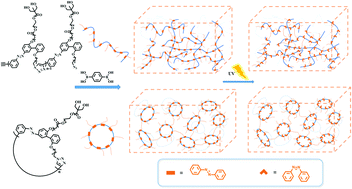
Cyclic architectures of polymers have shown unique properties or improved performance owing to the lack of chain ends. Herein, we describe the first example of azobenzene-induced photoresponsive gels based on cyclic polymers fabricated using an azide–alkyne and boronic acid-diol “click” reaction. The properties of cyclic gels with different cyclic polymer sizes were investigated by comparing them with those of linear gels made using polymeric linear precursors. It was observed that the cyclic topology exerted a larger influence on the properties of the gels. Further significant influences exerted by the topology were observed in cyclic gels with a smaller cyclic polymer size. Rheological measurements revealed that the cyclic gels have a higher storage modulus (G′) and loss modulus (G′′) compared to their linear counterparts, indicating that the former is stiffer than the latter. The gel-to-sol transition and photo-healing of the gels induced by azobenzene were observed by irradiation with UV/Vis light. Nevertheless, the cyclic gel with a smaller cyclic polymer size demonstrated a poor photo-responsive behavior owing to the restrictions of transformation of the trans–cis isomer of azobenzene moieties imposed by the cyclic topology. The results in this work showed that the network mesh space, and intramolecular and intermolecular interactions induced by the macrocyclic architecture endow the functional cyclic gels with unique properties.


 Please wait while we load your content...
Please wait while we load your content...