Bidirectional ROMP of paracylophane-1,9-dienes to tri- and penta-block p-phenylenevinylene copolymers†
Abstract
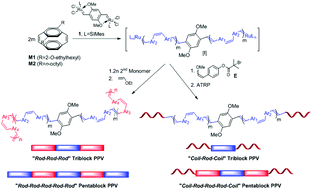
Dialkoxy and dialkyl substituted paracyclophane-1,9-dienes undergo bidirectional ring opening metathesis polymerisation (ROMP) on addition of bifunctional Hoveyda–Grubbs initiators. The living nature of the polymerisation was demonstrated for different [monomer]/[initiator] ratios and block copolymers were prepared by sequential ROMP and ROMP-ATRP to give fully conjugated rod–rod–rod and partially conjugated coil–rod–coil ABA and BAB triblock copolymers, respectively. These strategies were successfully extended to the synthesis of the corresponding pentablock copolymers. Optical and electrochemical properties of cis/trans and all trans homo and block copolymers are discussed.


 Please wait while we load your content...
Please wait while we load your content...