Polymerization of cystine-derived monomers
Abstract
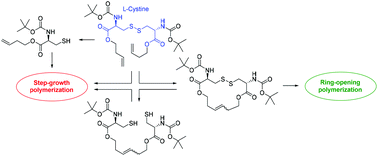
Cystine was used as a platform chemical to prepare cyclic and acyclic monomers for entropy-driven ring-opening polymerization (ED-ROMP) via olefin or disulfide metathesis and for step-growth polymerization. The olefin ED-ROMP of an olefin/disulfide containing 16-atom macrocycle using the 3rd generation Grubbs catalyst was examined in greater detail. Kinetic studies revealed that the catalyst turned inactive during the polymerization, which limited the achievable (apparent) polymer molar mass to ∼70 kg mol−1. Such limitation could be overcome with the disulfide ED-ROMP of the same macrocycle to yield polymers with molar masses of up to 180 kg mol−1. The step-growth polymerizations of acyclic diene and dithiol monomers via olefin metathesis or oxidation were far less effective and yielded just low molar mass polymers or oligomers; photopolymerization of a thiol–ene monomer produced a polyester with a molar mass of 35 kg mol−1.


 Please wait while we load your content...
Please wait while we load your content...