Polyaddition of vinyl ethers and phthalaldehydes via successive cyclotrimerization reactions: selective model reactions and synthesis of acid-degradable linear poly(cyclic acetal)s†
Abstract
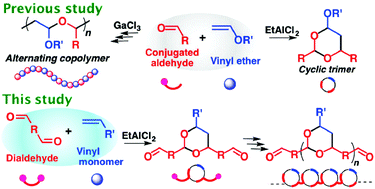
A polyaddition reaction via the cyclotrimerization of one vinyl ether and two conjugated dialdehyde molecules successfully proceeded using EtAlCl2 as a Lewis acid catalyst, yielding a polymer with cyclic acetal structures in the main chain. The use of a low-reactive or non-polymerizable vinyl ether and the choice of adequate catalysts were extremely important for the effective cyclotrimerization reactions. Diphenylethylene was also efficiently polymerized with dialdehydes. The polymers obtained by the polyaddition reaction showed selective acid-degradability and high glass-transition temperatures based on the cyclic acetal structures in the main chain.


 Please wait while we load your content...
Please wait while we load your content...