Diphenyl sulfone-based A–π-D–π-A dyes as efficient initiators for one-photon and two-photon initiated polymerization†
Abstract
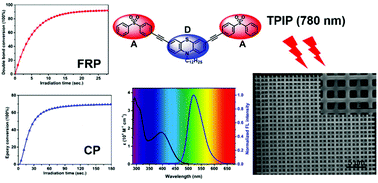
Three symmetrical quadrupolar A–π-D–π-A dyes, 3,6-bis[(p-phenylsulfonyl)phenylethynyl]-9-dodecyl-9H-carbazole (C-DSO), 3,7-bis[(p-phenylsulfonyl)phenylethynyl]-10-dodecyl-10H-phenothiazine (P-DSO), and 4,4′-bis[(p-phenylsulfonyl)phenylethynyl]triphenylamine (T-DSO) were prepared via Sonogashira coupling reaction. The proposed diphenyl sulfone-based dyes (DSOs) possess excellent light-harvesting capability and exhibit efficient intramolecular charge transfer character upon excitation. The two-photon absorption properties were evaluated by two-photon excited fluorescence spectrometry in the range of 720–850 nm. The maximum two-photon absorption cross-sections of C-DSO, P-DSO and T-DSO are 285, 1059, and 1030 GM, respectively. The two-component photointiating systems, DSOs/iodonium salt and DSOs/sulfonium salt, behave as efficient initiators for the cationic polymerization of epoxides and the free radical polymerization of acrylates under a 405 nm LED light source. In addition, two-photon initiated polymerization tests conducted on P-DSO and T-DSO indicate that the DSOs are promising initiators for 3D microfabrications. The underlying photochemical mechanisms were studied by steady state photolysis, electron spin resonance spin trapping, fluorescence spectroscopy, and cyclic voltammetry.


 Please wait while we load your content...
Please wait while we load your content...