Controlling silicone networks using dithioacetal crosslinks†
Abstract
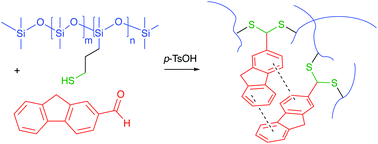
Traditional preparative methods for silicone elastomers center on platinum and tin-based cure chemistries. There is a need, for environmental and cost reasons, to find routes to silicones that do not require the use of heavy metals which remain in the elastomer after cure. This work describes dithioacetal silicone networks, which readily form between thiol-modified silicone monomers and polymers and aromatic aldehydes in the presence of acids, and do not require metal catalysts. Both chemical and physical crosslinks were utilized to independently manipulate elastomer properties. Subtle differences in the aromatic aldehyde used to crosslink the silicone led to large changes in modulus, due to differences in network structure. Using these associations, it was possible to tailor the silicone mechanical properties from very soft gels to much more rigid elastomers. The products were very stable to both hydrolytic and thermal stress, which was surprising given the normal susceptibility of silicones to acid-catalyzed reactions. The use of thioacetalization of readily available aromatic aldehydes as an alternative, metal free, organic cure provides an attractive route to polymer property optimization.


 Please wait while we load your content...
Please wait while we load your content...