Zwitterionic poly(sulfobetaine methacrylate)s in water: from upper critical solution temperature (UCST) to lower critical solution temperature (LCST) with increasing length of one alkyl substituent on the nitrogen atom†
Abstract
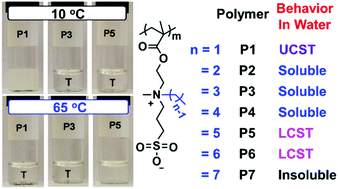
By gradually increasing the length of one linear alkyl substituent from one to seven carbon atoms on the nitrogen atom while keeping another substituent as a methyl group, poly(sulfobetaine methacrylate)s are found to exhibit a wide range of solution behavior in water, ranging from UCST, to totally soluble in water, to LCST, and then insoluble in water.


 Please wait while we load your content...
Please wait while we load your content...
