Facile preparation of stereochemistry-controllable biobased poly(butylene maleate-co-butylene fumarate) unsaturated copolyesters: a chemoselective polymer platform for versatile functionalization via aza-Michael addition†
Abstract
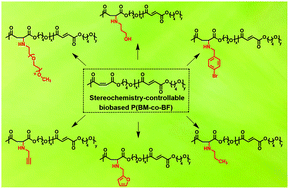
Maleate and fumarate-based unsaturated copolyesters are of interest as biomedical materials with tailorable properties. However, the preparation of unsaturated copolyesters with controllable maleate (cis) and fumarate (trans) isomer compositions is still a great challenge in polymer chemistry, because cis units always unbridledly isomerize to trans units in the polymerization process. Herein, we have presented a universal method for the facile synthesis of stereochemistry-controllable poly(butylene maleate-co-butylene fumarate) (P(BM-co-BF)) copolyesters. Then, we prepared a series of comparably high-molecular weight (Mw > 30 kDa) biobased copolyesters with a wide range of well-controlled cis–trans compositions. The solid-state microstructure, morphology, and crystallization behavior of P(BM-co-BF) copolyesters were systematically investigated to explore the respective effects of cis and trans isomers on the physical properties of copolymers. With the increment of the cis content, the copolyesters transform the phase state from semicrystalline to completely amorphous with tunable thermal and mechanical properties, which can be attributed to the conformational geometry effect. Consequently, the variation of the stereochemistry composition allows the physical properties to be tailored over a broad range. Furthermore, a merit of these copolyesters is to provide a chemoselective polymer platform for the introduction of various functional groups onto the backbone through a green aza-Michael addition reaction under extremely mild conditions, such as PEG, hydroxyl, alkynyl, etc. These incorporated functional groups can be used for the selective tailoring of the polyesters, which dramatically increase their potential for a wide variety of applications.


 Please wait while we load your content...
Please wait while we load your content...