Polarized olefins as enabling (co)catalysts for the polymerization of γ-butyrolactone†
Abstract
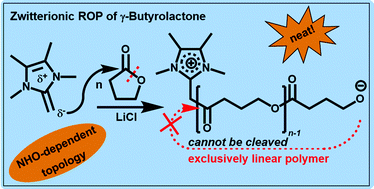
Suitable methods to polymerize γ-butyrolactone (GBL) by ring-opening polymerization (ROP) have emerged only recently. In view of the exciting properties of the corresponding aliphatic polyester, organocatalytic or simple metal-based catalyst systems are called for. To extend the currently limited set of suitable catalysts and to explore mechanisms beyond the “superbasic”, anionic pathway based on phosphazenes, so-called N-heterocyclic olefins (NHOs) have been employed for GBL homopolymerization. It was found that they can be applied in an organocatalytic setup but also in combination with lithium salts as cooperative Lewis pairs under bulk conditions (−36 °C). Metal-free setups succeed only in the presence of initiator (BnOH), whereby TONs of 140–230 have been realized by using the six-membered NHO 1,3-dimethyl-2-(1-methylethylidene)tetrahydropyrimidine (3, 70% conversion, 0.5 mol% NHO loading). MALDI-ToF MS analysis revealed that a mixture of cyclic and linear species is generated, whereby the proportion of cyclic material increases in the later stages of the polymerization. Conversely, when several NHOs were employed with lithium salts as Lewis pairs (NHO, LiX (X = Cl, I, OTf) and BnOH), the formation of macrocycles was found to be retarded. In the absence of protic initiator (BnOH) but presence of lithium salt, the polymerization mechanism can change from an anionic one to a zwitterionic ROP. As underlined by MALDI-ToF investigations, three different situations can occur, depending on the chemical structure of the NHO. Firstly, highly nucleophilic NHOs (7) will form stable zwitterionic species: the NHO is directly attached to the chain and cationizing it. As a bad leaving group, the NHO moiety cannot be substituted and exclusively linear poly(GBL) is generated. Less nucleophilic, sterically hindered NHOs (6) will still form zwitterions under these conditions, but substitution can occur, favouring a macrocyclic poly(GBL) topology. Finally, sterically encumbered but strongly basic NHOs (4) prefer enolization of the lactone over nucleophilic ring-opening, thus operating on an anionic polymerization pathway, comparable to phosphazene catalysis. Overall, the prepared poly(GBL) was found to be in the range of low polymeric/high oligomeric molecular weights (1000–9000 g mol−1, GPC) with the organocatalytic approaches delivering significantly higher conversion than reactions in the presence of lithium salts.


 Please wait while we load your content...
Please wait while we load your content...