Synthesis of block copolymers using end-functionalized polyacetylenes as macroinitiators†
Abstract
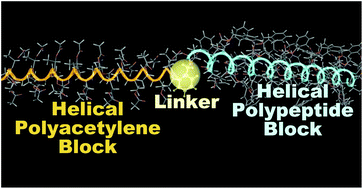
Polyacetylenes substituted with phenyl, N-tert-butoxycarbonyl-L-valine 4-anilide, and (S)-1-(acetoxy)ethyl groups with number-average molecular weights ranging from 3800 to 13 500 were synthesized by the polymerization of the corresponding acetylene monomers using [{1,1′-bis(diphenylphosphino)ferrocene}PdBr(C6H4-o-CH2OH)] as a catalyst. L-Lactide and γ-benzyl-L-glutamate N-carboxyanhydride were polymerized using the polyacetylenes as macroinitiators to obtain the block copolymers, some of which formed chiral secondary structures. A block copolymer consisting of helically twisted polyacetylene and α-helical peptide was successfully synthesized for the first time.


 Please wait while we load your content...
Please wait while we load your content...