Ultrafast and efficient aza- and thiol-Michael reactions on a polyester scaffold with internal electron deficient triple bonds†
Abstract
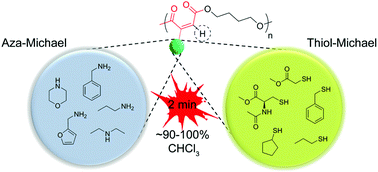
A polyester scaffold possessing electron deficient triple bonds in the main chain was prepared and utilized as a precursor for aza- and thiol-Michael addition reactions. A variety of primary and secondary amines as well as thiol compounds were utilized in the reactions. Very high efficiencies were found for all Michael addition reactions in a reasonably short time (2 min). While aza-Michael addition reactions do not require any catalysts, thiol-Michael addition reactions could be performed in the presence of a catalyst. After a detailed catalyst search, 1,4-diazabicyclo[2.2.2]octane (DABCO) was found to be the most efficient catalyst for thiol-Michael addition reactions. It is also observed that when amidine and guanidine bases were utilized for thiol-Michael addition reactions, a second thiol addition appreciably occurred on the remaining double bonds. Besides, for the first time, one-pot and one-step heterofunctionalization on the polyester was performed either solely by aza-Michael addition reactions employing three or four different secondary amines, or by a combination of aza- and thiol-Michael addition reactions.


 Please wait while we load your content...
Please wait while we load your content...