Polymerization based on alternating insertion of an isocyanide and alkyne into palladium–carbon bonds†
Abstract
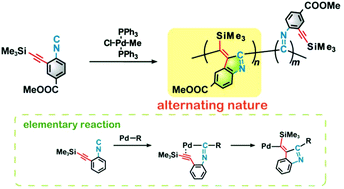
The first polymerization system based on the alternating insertion of an isocyanide and alkyne into palladium–carbon bonds has been developed. An aryl isocyanide containing isocyanide and alkyne moieties in a single molecule was polymerized using chloro(methyl)bis(triphenylphosphine)palladium as a catalyst. The resulting polymer was characterized by IR and NMR spectroscopic analyses, which showed that the polymerization reaction proceeded through the intramolecular alternating insertion of an isocyanide and alkyne to produce indole units in the main chain.


 Please wait while we load your content...
Please wait while we load your content...