Thionating iso-diketopyrrolopyrrole-based polymers: from p-type to ambipolar field effect transistors with enhanced charge mobility†
Abstract
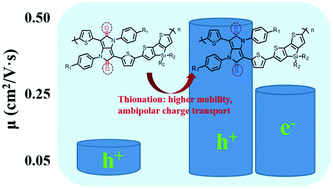
In this paper, two donor–acceptor-type π-conjugated polymers (P1 and P2) with 4,4′-bis(2-ethylhexyl)-5,5′-bis(trimethyltin)dithieno[3,2-b:2′,3′-d]silole as the donor and iso-diketopyrrolopyrrole or iso-dithioketopyrrolopyrrole as the acceptor moiety were designed and synthesized via palladium-catalyzed Stille coupling, and their optical, electrochemical and organic field effect transistor (OFET) properties were investigated. P2 could be regarded as the simple thionated product of P1, however, only one atom difference has resulted in their significantly different optical and electronic properties. P2 exhibited an obviously bathochromic shift absorption compared to P1. Noticeably, P1 only showed a hole mobility of 0.09 cm2 V−1 s−1, while P2 exhibited hole and electron mobilities of up to 0.49 cm2 V−1 s−1 and 0.26 cm2 V−1 s−1, respectively, which indicated that the thionation could not only improve charge mobility but also give the polymer ambipolar transporting properties. Quantum chemical calculations and electrochemical analysis revealed that the thionation could further optimize the frontier molecular orbitals and facilitate the charge injection. This work demonstrated that iso-dithioketopyrrolopyrroles could be promising building blocks for high-performance organic field effect transistors.


 Please wait while we load your content...
Please wait while we load your content...