Efficient polymerization and post-modification of N-substituted eight-membered cyclic carbonates containing allyl groups†
Abstract
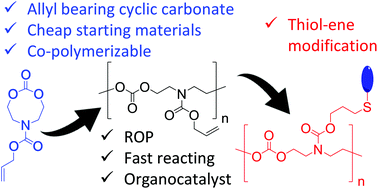
Aliphatic polycarbonates are promising materials in the biomedical field due to their low toxicity, biocompatibility, and biodegradability. A popular approach in obtaining these materials is through the organocatalyzed ring-opening polymerization (ROP) of cyclic carbonates. Functional polycarbonates can be obtained by (co)polymerizing allyl-functional cyclic monomers, which can be chemically modified via radical thiol–ene click reactions after the ROP process. To date, allyl-bearing 6-membered cyclic carbonates have been reported, however their polymerization kinetics are slow. In previous works, larger cyclic carbonates (e.g. N-substituted eight membered cyclic carbonates) have demonstrated superior reactivities over their smaller counterparts and hence, in this work, we investigated the preparation and ROP of two allyl bearing N-substituted eight membered cyclic carbonates. One of these monomers, with a pendent carbamate group, displayed substantially enhanced polymerization kinetics that allowed for efficient homopolymerizations and co-polymerizations with commercially available monomers (e.g. trimethylene carbonate, or TMC). Lastly, the polymers underwent almost quantitative post-polymerization modification via thiol–ene chemistry to yield different functionalized polycarbonates. We also demonstrated that the post-polymerization reaction could be used to form polycarbonate-based gels with multifunctional thiols.
- This article is part of the themed collection: Polymer Chemistry Lectureship Winners


 Please wait while we load your content...
Please wait while we load your content...