Kinetic aspects of formation and processing of polycaprolactone polyurethanes in situ from a blocked isocyanate†
Abstract
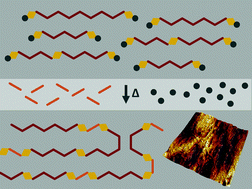
In order to produce segmented polyurethane polymers (PU) that can be processed readily into elastomeric thin films, a thermally labile blocking agent was used to form an isocyanate prepolymer and prevent reaction with moisture while facilitating the formation of the polymers by bulk thermal polymerization. The approach is to react a polymeric diol and isocyanate to form a prepolymer, end cap or ‘block’ the isocyanate, combine it with a chain extender, and form films that then thermally polymerize when the blocking agent dissociates and evaporates. A major advantage of this process is that the blocked isocyanate prepolymer is completely stable and unreactive on exposure to incidental moisture. Film polymerization kinetic studies were performed with the blocked isocyanate at a series of temperatures and reaction times. The extent of reaction was followed by proton NMR and the molecular weights of the resulting films were characterized by gel permeation chromatography (GPC). The data indicate how both temperature and reaction time are important for the formation of high molecular weight polyurethanes and that a catalyst is required to facilitate the reaction. Films with high molar masses and excellent mechanical properties were obtained. High resolution quantitative atomic force microscopy (AFM) imaging disclosed interesting features of the polymer morphology at the nanoscale that relate directly to the polymer mechanical properties.


 Please wait while we load your content...
Please wait while we load your content...