A hyperbranched amphiphilic acetal polymer for pH-sensitive drug delivery†
Abstract
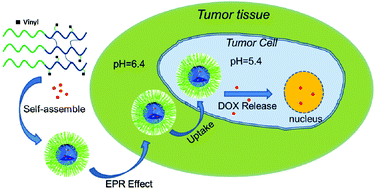
Nanoparticles are appealing drug delivery systems since they promise prolonged circulation time and predictable release behaviors. The current work reports a novel hyperbranched amphiphilic block copolymer synthesized using deactivation-enhanced atom transfer radical polymerization (DE-ATRP) for smart drug delivery. PEG2000-Br was applied as a macroinitiator to initiate acid-cleavable divinyl (ACD) monomers linked by acetal groups, formulating the hydrophilic (PEG) and hydrophobic (ACD) segments. The polymer appeared to self-assemble into micelles with diameters in the range of 70–100 nm, and was examined for the controlled release of doxorubicin (DOX). Results showed that DOX-loaded micelles (∼90 nm) could achieve drug loading as high as 8.2 wt%, with interesting pH-dependent release behaviors. Studies with flow cytometry (FCM) and confocal laser scanning microscopy (CLSM) showed that DOX-loaded micelles exhibited a high cellular uptake performance by HeLa cells, which indicated promising anti-tumor efficacy for such a drug delivery system. Additionally, such DOX-loaded micelles exhibited remarkable cytotoxicity against HeLa cells in a dose- and time-dependent manner due to the enhanced cell uptake behavior of micelles. These results indicated that the polymeric micelles might be used as a promising candidate for a pH-responsive drug delivery for cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...