Synthesis of enzyme-responsive phosphoramidate dendrimers for cancer drug delivery†
Abstract
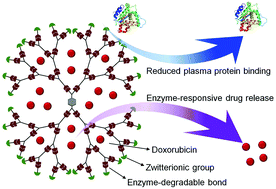
Stimuli-responsive dendrimers are attractive nanocarriers for cancer drug delivery due to their inherent structure, biodegradability and precise control of release of a payload in response to specific triggers. However, the efficient and precise synthesis of such dendrimers with site-specific responsiveness is challenging. We report an efficient synthesis of enzyme-responsive phosphoramidate (PAD) dendrimers from a pair of monomers using click and click-like reactions. These dendrimers were stable in phosphate buffered saline (PBS) but degradable in the presence of phospholipase C (PLC) that was overexpressed on cancer cells. The surface modification of the dendrimers with zwitterionic groups (2-methacryloyloxyethyl phosphorylcholine, MPC) greatly reduced the nonspecific binding of the plasma proteins and therefore enhanced blood circulation time. The PAD-MPC dendrimers easily encapsulated hydrophobic drugs such as doxorubicin (DOX). The DOX-loaded PAD-MPC (PAD-MPC/DOX) showed PLC-responsive drug release and efficient delivery of DOX to cancer cells by overcoming multidrug resistance. The PLC-responsive PAD-MPC/DOX was highly toxic to cancer cells and less effective on normal cells. The PAD-MPC/DOX showed improved therapeutic effects and reduced toxicity in athymic nude mice bearing xenografts of MCF-7/ADR breast cancer.


 Please wait while we load your content...
Please wait while we load your content...