Nonstoichiometric polycondensation based on Friedel–Crafts acylation in superacids for the syntheses of aromatic polyketones†
Abstract
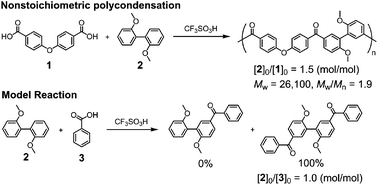
Based on Friedel–Crafts acylation, nonstoichiometric polycondensation was achieved using 4,4′-dicarboxydiphenyl ether and 2,2′-dimethoxybiphenyl in trifluoromethanesulfonic acid. The decrease in the acylation reactivity of 2,2′-dimethoxybiphenyl by protonation and the increase in the acylation reactivity of the monoacylated species by deprotonation play a key role in successful nonstoichiometric polycondensations.


 Please wait while we load your content...
Please wait while we load your content...