A novel radical polymerization system initiated by a redox reaction with NHPI and xanthone†
Abstract
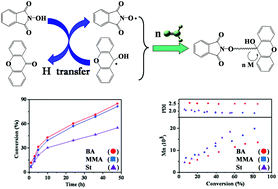
A novel redox reaction system consisting of xanthone (XT) and N-hydroxyphthalimide (NHPI) for radical polymerization is developed where NHPI and XT experience a one-electron-transfer reaction, which produces two kinds of radicals, PINO radicals and cycloketyl (CK) radicals. This redox system is efficient to initiate the radical polymerization of three typical monomers, i.e., methyl methacrylate (MMA), butyl acrylate (BA) and styrene, and the analytical results of the end-groups by NMR and MALDI-TOF MS demonstrate that PINO radicals initiate the polymerization, while CK radicals act as dormant radicals. As the polymerization proceeds, a significant increase in molecular weight and a decrease in PDI are observed, which could be attributed to the reversible deactivation between CK radicals and polymer chain radicals.

 Please wait while we load your content...
Please wait while we load your content...