Facile synthesis of poly(vinyl acetate)-b-polystyrene copolymers mediated by an iniferter agent using a single methodology†
Abstract
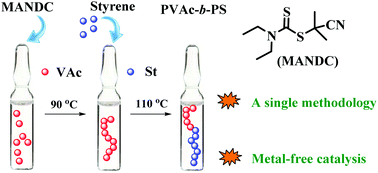
The preparation of well-defined block copolymers from both non-conjugated and conjugated monomers is still challenging. In this work, a typical block copolymer poly(vinyl acetate) (PVAc)-b-polystyrene (PS) was successfully synthesized by using a successive iniferter strategy. Herein, PVAc was first obtained in the presence of an iniferter agent (e.g., 1-cyano-1-methylethyldiethyldithiocarbamate (MANDC), 2-(N,N-diethyldithiocarbamyl)-isobutyric acid ethyl ester (EMADC) or 2-(ethoxycarbonothioyl)sulfanyl propanoate (EXEP)). Then the resultant PVAc with high end-group functionalities could be used as an effective macroiniferter agent for the polymerization of styrene (St). The kinetic studies of the chain extension experiment with St indicated the “living” features of the polymerization system. In addition, the obtained PVAc-b-PS copolymers could be easily converted into PVA-b-PS amphiphiles by methanolysis of the poly(vinyl acetate) block. Importantly, the synthesis of the copolymers just uses a single simple and metal-free catalyzed polymerization method, which may be of great potential for industrial production.


 Please wait while we load your content...
Please wait while we load your content...