Synthesis of cinnamoyl and coumarin functionalized aliphatic polycarbonates†
Abstract
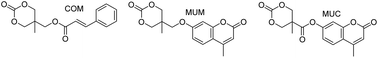
With the objective of generating photo-responsive polymers, carbonate monomers with pendant cinnamoyl or coumarin moieties, which are capable of photo-reversible dimerization, were synthesized. The pendant groups were attached via ester and ether functional groups, which affected both the ease of preparation and the stability of the final monomers. In addition, the homopolymerization kinetics of the various monomers were examined using several catalysts. The presence of esters in the pendant groups enhanced the polymerization rate of the functionalized monomers relative to trimethylene carbonate (TMC) under the same conditions, with up to 60% monomer conversion in an hour relative to 5% for TMC. The copolymerization of these functionalized monomers with TMC and ε-caprolactone was demonstrated using a variety of catalyst systems and the end group fidelity and molecular weight control of the various polymerizations were compared. Both the cinnamate functionalized monomer and an ether-linked coumarin functionalized monomer copolymerized well by catalyst-free melt polymerization while an ester-linked coumarin functionalized monomer underwent transesterification side-reactions with all catalyst systems examined except triflic acid. Finally, the use of these photo-responsive copolymers to successfully stabilize polymersome membranes was demonstrated.


 Please wait while we load your content...
Please wait while we load your content...