The effect of conjugated π-bridge and fluorination on the properties of asymmetric-building-block-containing polymers (ABC polymers) based on dithienopyran donor and benzothiadiazole acceptors
Abstract
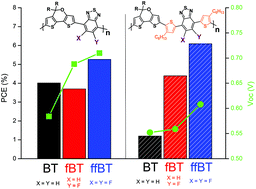
To investigate the effect of conjugated π-bridge and fluorination on the properties of asymmetric-building-block-containing polymers (ABC polymers), hexylthiophene (HT) was inserted into D–A-type polymers based on asymmetric dithieno[3,2-b:2′,3′-d]pyran (DTPa) donor and benzothiadiazole (BT), mono-fluorinated benzothiadiazole (fBT) or di-fluorinated benzothiadiazole (ffBT) acceptors, respectively. In comparison with non-bridged analogs, introducing the HT π-bridge between DTPa and BTs significantly reduced the intramolecular charge transfer and consequently affected the absorption spectra, frontier energy levels, and electrochemical and charge transport properties of the resulting ABC polymers. Interestingly, HT π-bridge enlarged the fluorination effect on photovoltaic properties, and the power conversion efficiency (PCE) increased gradually from 1.56% (DTPa-HTBT) to 4.39% (DTPa-HTfBT) and 6.09% (DTPa-HTffBT), significantly different with non-bridged polymers. Most importantly, with the increase in the number of the fluorine atoms on the BT unit, all of the photovoltaic parameters, including PCE, Voc, Jsc, and FF, improved gradually, which provides a useful guide for the further design of ABC photovoltaic polymers.


 Please wait while we load your content...
Please wait while we load your content...