Kinetic and mechanistic investigation for the copolymerization of CO2 and cyclohexene oxide catalyzed by trizinc complexes†
Abstract
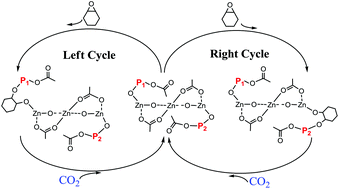
The trizinc complexes Zn3(OAc)4(Ln)2, 1a and 1b, which were coordinated with Schiff-base ligands via a simple and economical method, have been examined and found to be highly effective and efficient toward the copolymerization of CO2 and cyclohexene oxide (CHO). In this work, the kinetics for the copolymerization of CO2 and CHO using 1b as the catalyst was monitored via in situ ATR-FTIR spectroscopy. The reaction orders’ dependencies on catalyst concentration, initial CHO concentration and CO2 pressure, as well as activation energies (Ea) of polycarbonate and cyclic carbonate formation, were investigated in detail. The practical amount of active zinc sites in the copolymerization was calculated using the parameters obtained from 1H NMR and GPC measurements. The initiating reaction details were simulated using density functional theory, and the potential energy surfaces were obtained. Based on the results of all characterizations and kinetic investigations, a unique initiating reaction and copolymerizing mechanism were proposed when using Zn3(OAc)4(Ln)2 as the catalyst.


 Please wait while we load your content...
Please wait while we load your content...