Selective monophosphorylation of chitosan via phosphorus oxychloride†
Abstract
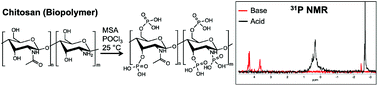
Chitosan was selectively monophosphorylated via reaction with phosphorus oxychloride (POCl3) to enhance water solubility while avoiding polyphosphate formation. The use of POCl3 resulted in negligible product degradation (i.e., breakdown of O-glycosidic bonds) even after a 3 d reaction period (<5% weight loss). X-ray photoelectron spectroscopy (XPS) characterization of the POCl3-phosphorylated chitosan (P-chitosan) revealed a phosphorus to nitrogen (P/N) atomic ratio of 0.30. Phosphorus-31 nuclear magnetic resonance (31P NMR) spectroscopy verified the monophosphorylation of chitosan's primary and secondary alcohols, and primary amines. The calcium chelation efficiency for the phosphorylated product approached 0.05 mg Ca2+ per mg of P-chitosan as measured by inductively coupled plasma-optical emission spectrometry (ICP-OES), indicating improved chelation over native chitosan. This selective monophosphorylation approach proved useful for modifying other biopolymers, including cellulose and alginate.


 Please wait while we load your content...
Please wait while we load your content...